What is GammaTile Therapy?
February 3, 2021
Frequently Asked Questions
How does GammaTile Therapy work?
GammaTile is a Surgically Targeted Radiation Therapy (STaRT) for operable brain tumors that provides immediate radiation treatment after tumor removal. Each GammaTile has radiation sources embedded in a collagen tile that deliver a precise dose of radiation focused right where it is needed and away from healthy brain tissue. In a clinical study, this resulted in nearly twice as many tumor-free months compared to the patients’ most recent prior same-site treatment.[1] Learn more about the clinical study.
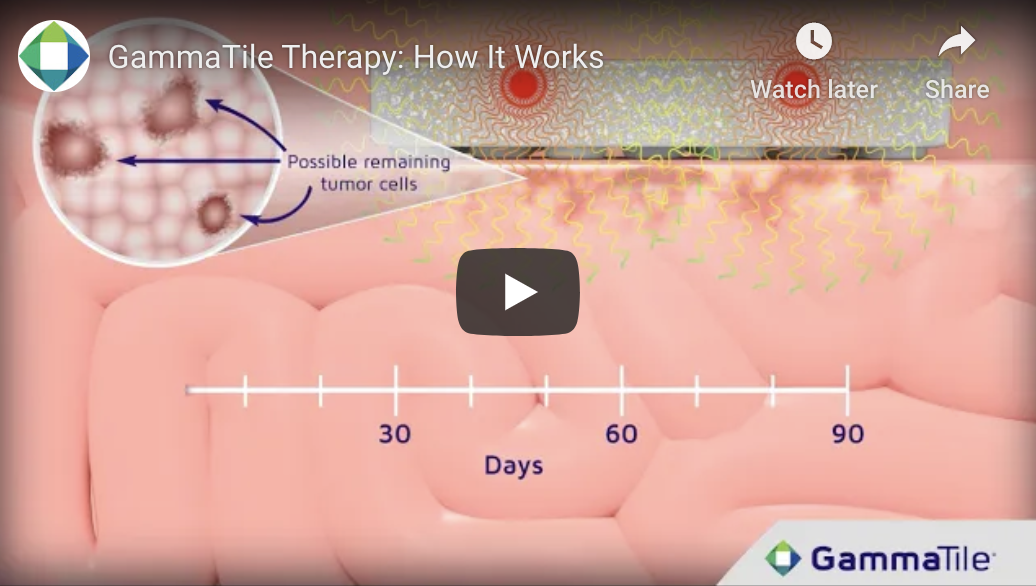
How does GammaTile Therapy work?
The neurosurgeon places the GammaTile(s) precisely where treatment will help the most—immediately after tumor removal. Like other radiation therapies, GammaTile Therapy works by disrupting the tumor cell replication process. Radiation damages the tumor cell DNA, so the cell is unable to replicate and eventually dies. The collagen tile keeps the radiation sources in place while the radiation is being released. Over time, the body naturally absorbs the collagen tile.
Is GammaTile FDA approved?
GammaTile Therapy is FDA cleared to deliver radiation therapy for patients with newly diagnosed malignant brain tumors and recurrent brain tumors.
Do I have to stay in the hospital longer after surgery?
No, typically there is no need to extend your hospital stay or to travel for additional, ongoing radiation treatments.
If I have had radiation therapy in the brain previously, can I still have GammaTile Therapy?
In a recent clinical study, all patients treated with GammaTile Therapy had previously had radiation therapy in the brain.[1] However every situation is unique. Together with your care team, your radiation oncologist will review your previous records to determine if GammaTile Therapy might be right for you.
What are the side effects of GammaTile Therapy?
Compared to other radiation treatments, GammaTile Therapy side effects are typically fewer.[2] The potential for adverse events depends on the radiosensitivity of the exposed tissue, the amount of radiation delivered, and the placement of GammaTile(s).
Because GammaTile is placed during tumor removal surgery, the possible complications of neurosurgery may also apply; including, but not limited to, cerebrospinal fluid leaks, infection, delayed hemorrhage, seizures, and adhesion formation. For more information about potential side effects, talk to your healthcare provider.
Find more answers and resources about GammaTile therapy at https://www.gammatile.com/education.
REFERENCES
1. Nakaji P, Youssef E, Dardis C, Smith K, Pinnaduwage D, Brachman D. Surgically targeted radiation therapy: a prospective trial in 79 recurrent, previously irradiated intracranial neoplasms. Poster presented at: 2019 AANS Annual Scientific Meeting; April 2019; San Diego, CA.
2. Brachman D, Youssef E, Dardis C, Smith K, Pinnaduwage D, Nakaji P. Surgically targeted radiation therapy: Safety profile of collagen tile brachytherapy in 79 recurrent, previously irradiated intracranial neoplasms on a prospective clinical trial. Brachytherapy. 2019;18(3):S35-S36.